Osteonecrosis of the Femoral Head (Avascular necrosis)
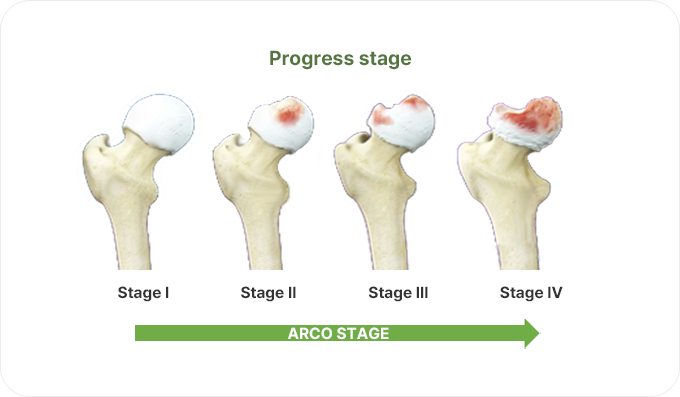


This is a disease in which blood flow to the upper end of the thigh bone (femoral head) is blocked and bone tissue dies.The incidence is relatively high in young people in their 30s to 50s, and it is reported more often in men than in women.When the artificial hip joint replacement is performed, the decline in quality of life is inevitable, so there is a high demand for preserving the patient's own bones.In the United States, it is estimated that 20,000 to 30,000 new patients occur every year, and in Korea, it is also reported that 2,000 to 3,000 patients occur annually.
In particular, asthe steroids are widely used as treatments for COVID19, SARS, etc., the incidence of femoral head osteonecrosis is rapidly increasing.Therefore, WHO officially recommends the early MRI diagnosis for the patients who have undergone such treatments.(Source: Zhang B et al JBMR 2020; Yu EW et al JBMR 2020; Patel MS et al JBJS Review 2020)
· Excessive drinking
· Steroid preparations
· Complex chronic diseases
· Nutritional deficiencies
· Aging
When this disease develops as the progressive disease, improvement is difficult, and the surgery to replace the joint with an artificial joint (Total Hip Arthroplasty, THA) is the only alternative.Initially, there are no special symptoms, but it gradually expands to severe pain in the inguinalregion, making it difficult to walk or sit cross-legged.
| Basic research | Non-clinical | Phase 1 | Phase 2 | Phase 3 | Permission |
|---|---|---|---|---|---|
|
Phase 2 clinical trial
|
Core decompression is the procedure that reduces pain by opening an access route to the necrotic area (femoral head) on the outside of the patient's thigh, thereby lowering the increased internal pressure in the necrotic area of the bone head, normalizing the blood flow within the bone head, and promoting the revascularization.However, because it is not the fundamental treatment method, the disease continues to progress and eventually requires theTotal hip arthroplasty.During the core decompression procedure, the osteonecrosis area is removed, and the cell therapy agent (CF-M801) is injected into the lesion to induce the autogenous bone production and inflammation relief.
CF-M801 at three different doses was transplanted into theRat model with Tibia defects, and non-toxicity and non-toxic doses were verified with cells, resulting in the non-toxic conclusion.
Rat model with defective Tibia was followed for 26 weeks and then analyzed for toxicity and tumorogenicity.As a result, no toxicologically deleterious changes or tumor formation were observed.
After CF-M801 was administered to theRat model with defective Tibia, its retention and distribution in the body were confirmed in all organs.After 1 week, no administered cells were found in any organ except the administration site, and after 14 weeks, no administered cells were found in all organs.
After transplanting CF-M801 into theRadial defect Rat model, bone regeneration ability was verified through X-ray and micro-CT analysis.
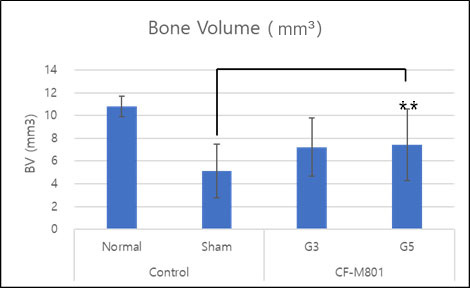
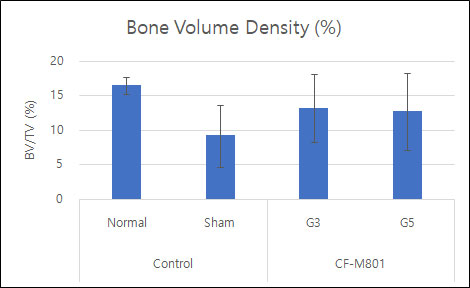
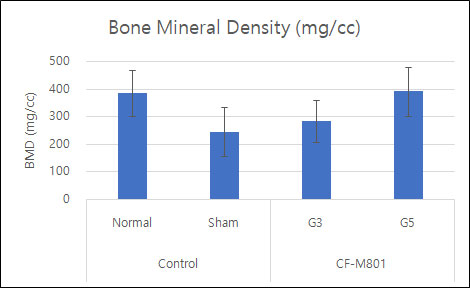
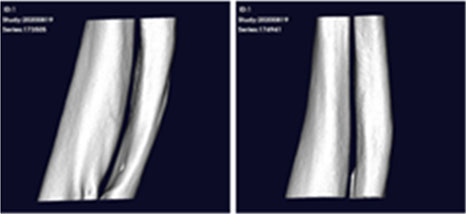
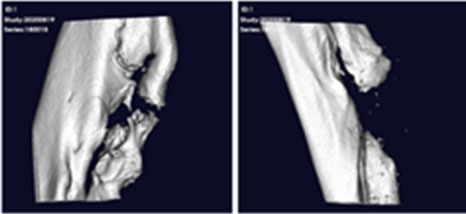
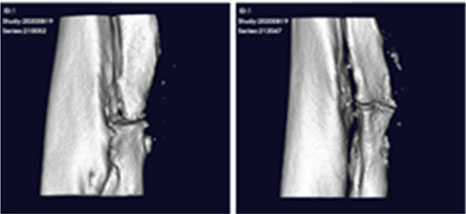
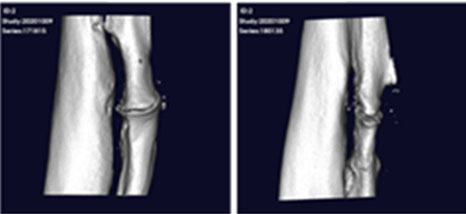
Even in the case of quantitative analysis, CF-M801 showed better indicators such as Bone Volume, Bone Volume Density, and Bone Mineral Density compared to the Sham control group.Additionally, the result of both P5 and P7 were excellent.Therefore, it is believed that CF-M801 shows the excellent therapeutic effects without being significantly affected by the number of cell passages, at least between P5 and P7.
CF-M801 was transplanted into the femur defect area of the goat and analyzed in various ways through micro-CT and histological evaluation.As a result of micro-CT analysis and tissue slide analysis of 12 and 26 weeks after cell transplantation, it was confirmed that the bone regeneration efficiency was excellent.
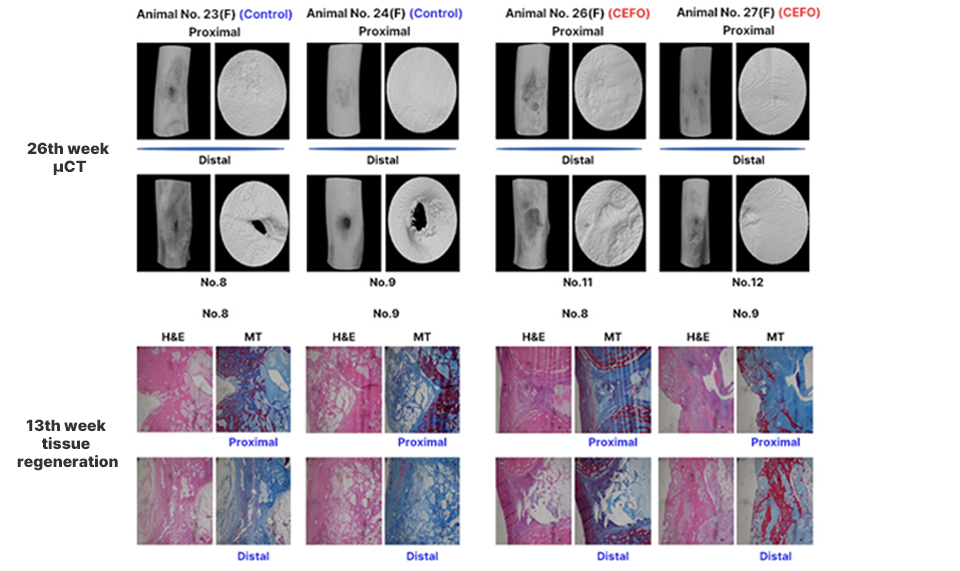
<Analysis of Micro CT image and tissue slide>
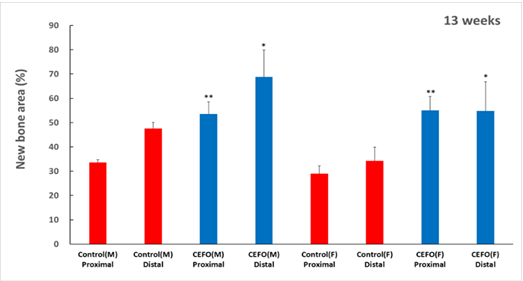
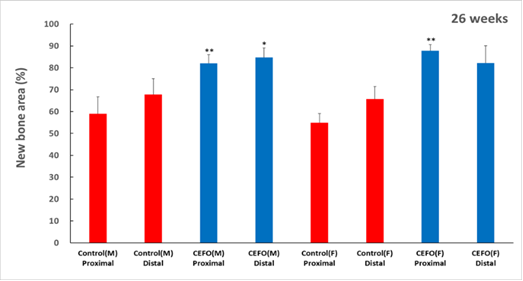
<New Bone Area at 13 and 26 weeks after cell therapy agent transplantation>
Evaluation of Safety and Efficacy of Cell Therapy Based on Osteoblast Derived from Umbilical Cord Mesenchymal Stem Cell for Osteonecrosis of the Femoral Head: Study Protocol for a Single-Center, Open-Label, Phase I Clinical Trial
2021, Research Square
Heejae Won, Shin-Yoon Kim, Sunray Lee, Hyun Sook Park, Seung-Hoon Baek